In the
process of acquiring a couple of Everyland magazines with articles by the author Verrill, I noticed this magazine in the vendor’s virtual storefront. I thought it might
be interesting so…/drf
The Story of Aluminum
 From the
magazine, Industrial Arts and
Vocational Education March,
1931. Digitized by Doug Frizzle, June 2012.
From the
magazine, Industrial Arts and
Vocational Education March,
1931. Digitized by Doug Frizzle, June 2012.
READ IT TO YOUR STUDENTS —Inspirational
—Tells How a Typical American Boy Achieved Fame and Riches.
“PROFESSOR—I've got it!" exclaimed a
young man, bursting excitedly into the
private laboratory of the head of the chemistry department of an Ohio college, on the
morning of February 23, 1886.
Extending his cupped hand, he
proudly exhibited to the astonished
professor, half a dozen little globules of a silver-colored metal.
It was no wonder he was
excited, or that his excitement was shared by the
incredulous professor, for at 22 years of age he had achieved what some of the
world's greatest scientists had failed to achieve after half a century of
application to the same problem. And
where they had enjoyed the facilities of great laboratories, with all
manner of scientific equipment, and yet had failed, this 22-year-old American
youth had worked out the problem in the woodshed in his backyard, with the crudest kind of equipment.
IT all came about as the result of a chance remark made by Frank Fanning
Jewett, his chemistry professor at Oberlin
College, one morning
during the young man's senior year.
In talking with the students in his
chemistry class Prof. Jewett made the
offhand observation that if anyone should invent a process by which aluminum
could be made on a commercial scale,
he would not only be a benefactor to the
world, but he would also be able to lay up for himself a great fortune.
Serious-minded Charles Martin
Hall nudged a classmate and whispered, "I'm going for that metal."
Go for it he did, and in dead
earnest. Discovering a cheap method of producing aluminum became an obsession
with him. Had he known that he was racing with another
young man, across the seas, and had
he realized how nearly the two would
tie each other, he would scarcely
have slept during the next year! As
it was, he spent much of his spare time outside of school hours in the college laboratory working doggedly on the problem, for he had read somewhere,
that every clay bank was potentially a mine of aluminum, and that the metal was as costly as silver.
But all his application, and
all his admitted genius, and all his knowledge of chemistry, seemed of no avail
against this aluminum riddle. "Going for it" was one thing, but
getting it was quite another. All
avenues seemed to lead to failure.
It was not until eight months
after he had finished college that he started thinking along an entirely new
line. If aluminum could not be extracted inexpensively by the chemical processes with which scientists had
been struggling for half a century, and with which he had worked unsuccessfully
for so many months, might it not be done by electricity?
That was a new idea. It seemed
worth investigating.
Hall knew something of electrolysis and he lost no time in fitting up the
woodshed behind his father's house
on East College Street,
Oberlin, as a laboratory. Borrowing an odd assortment of battery jars and
plates from the
college laboratory, and buying a small crucible, he set them
along the edge of the upper floor of the
two-story woodshed, so that he could stand while at work, on the lower steps and oversee both levels. Then he set
up a homemade furnace and bellows.
Days were spent—precious days
that were worth hundreds of thousands of dollars to him had he known it—in
constructing crude pieces of apparatus that he had not the
small means to purchase.
At last, however, everything
was ready for an entirely new experiment.
Melting some cryolite in his little clay crucible, and
dissolving some "alumina"
(refined aluminum ore) in it, he switched on his batteries and passed the electric current through the
molten mass for about two hours.
We can imagine "with
what impatience he paced up and down in the
woodshed during those two hours!
Finally the time was up and he poured out the molten mass. Alas! There was no aluminum!
However, he was not
discouraged. Indeed, it is said of Charles Martin Hall that he was never
discouraged for more than a few hours at a time.
He started to think his way
into the problem, and it may be
observed in passing that Hall never worked at random
nor never stumbled onto things— he thought things through. In this instance, he
came to the conclusion that there might be some
impurities in his clay crucible and they
might be affecting the result.
Promptly
he constructed a carbon lining for the
crucible and he proceeded to repeat the
experiment.
Again he waited impatiently
for two hours as the current from his makeshift batteries passed through the molten mass.
Could he believe his eyes?
There, in the bottom of the
crucible, were a number of small globules of aluminum!
Excitedly he called his
sisters and showed them the still hot little "buttons" of
aluminum. (Some of these are today carefully preserved in the Pittsburgh
offices of Aluminum Company of America.)
As soon as these globules, or "buttons," were cool
enough to handle, Hall took them in the palm of his hand and hurried to the college, where he burst in upon Professor Jewett
with the startling news with which
this story opens "Professor—I've got it!"
That day—February 23, 1886—marked
the birth of a new metal age— The Age
of Aluminum!
Two months later to the day (April 23, 1886) a French chemist, Paul L.
T. Heroult (by coincidence also 22 years of age) applied for a French patent on
the identical process!
Hall had won by a margin of
eight weeks!
A New Industry Is Born
CURIOUSLY enough, after a company had been organized to produce aluminum by
Hall's process, one of the first
articles made was a teakettle. But this was not chance. Because it was known
that aluminum conducted heat so efficiently, and because it was so easy to keep
clean and bright, and because it promised
to wear forever, Hall and his backers saw that it was an ideal metal for
cooking utensils. Thus it was that aluminum got its first big start in the kitchen.
Everybody knows how aluminum
cooking utensils took America
by storm, and how much they have
lightened the drudgery of housework
and added to the cleanliness and
cheerfulness of our kitchens and the
wholesomeness of our meals.
But the
usefulness of aluminum was not to be confined to the
kitchen. It was destined to invade hundreds of industries and to be used for
thousands of purposes.
For example, because of its
light weight and excellent electrical conductivity, it began to be used to make
cable (with a core of steel wire for greater strength) for conveying electrical
current across country. Thousands of miles of aluminum power lines are to be seen
today.
When the
automobile came along a dozen new
uses were found for this light metal which automotive
engineers found saved weight and made possible speedier cars. Pistons and
connecting rods of aluminum alloys became almost standard in automobile engineering practice. Today, eight out of
ten motor car manufacturers use aluminum alloy pistons in their motors.
Close on the heels of the
automobile came the airplane, and here aluminum figured from the
first as an essential metal. It is the
cheapest metal that is light, and at the
same time strong enough to stand the
stresses and strains of air navigation.
However, these modern miracles of metallurgy were not accomplished with the
quality of aluminum which Hall had made in his woodshed laboratory. When
aluminum began to be used for industrial purposes, it was found that for some uses it lacked the
necessary strength. A research laboratory was established and presently, by combining aluminum with small percentages of other metals, a series of "alloys" was
developed. After these alloys had
been heat treated they became
exceedingly tough and strong —some
of them as strong as structural
steel.
 These alloys contain 95 per
cent or more of aluminum, so they
preserve all the characteristic
lightness and bright color of the
parent metal, but they are many
times as strong and are now used for the
heaviest kind of duty. For example, the
bodies of some of the armored motor cars seen on our city streets, are
made of strong aluminum alloys. Could any but a stout metal be used for such a
purpose?
These alloys contain 95 per
cent or more of aluminum, so they
preserve all the characteristic
lightness and bright color of the
parent metal, but they are many
times as strong and are now used for the
heaviest kind of duty. For example, the
bodies of some of the armored motor cars seen on our city streets, are
made of strong aluminum alloys. Could any but a stout metal be used for such a
purpose?
When railroad and street car
companies began to face the necessity of lighter cars and locomotives, so that their
trains and trolleys might run on faster schedules to meet the demand for greater speed in transportation, they turned naturally to these
strong alloys of aluminum. Roofs, frames and side walls of passenger cars made
of rolled sheets and structural shapes of strong aluminum alloys. Drop-forged
aluminum driving-rods for connecting the
huge driving-wheels of powerful steam locomotives
are already beginning to be used, and the
time seems not far distant when aluminum will play a big part in speeding up our
train and trolley service all over the
country, at the same time reducing
operating costs for the
transportation companies.
From
year to year, many other interesting
discoveries were made about aluminum. It was found that this bright metal could
be ground to a flaky powder and made into paint. At first this flaky powder was
mixed with banana oil and used almost exclusively for painting radiators.
But soon paint makers found
that it could be mixed with varnish, like any other
paint pigment. When this aluminum paint is applied, the
minute flakes of metal overlap one another
(as revealed by microscopic examination), and form a tough and flexible
metallic coat that defies rain, sun, snow or hail.
This was an important
discovery, for while people may not want aluminum-colored houses, it has been
demonstrated that aluminum paint as a priming coat forms a thin film of metal
that protects the wood underneath
against moisture changes, and makes the
color coats that are put on top wear much longer. Eventually, it is thought, the best grades of lumber may come already "primed" with aluminum paint.
Another
interesting discovery was that aluminum could be rolled into foil—some of it so thin that it would take ten sheets to
equal the thickness of the paper on which this story is printed!
Because this foil is pure and
clean and will not tarnish, and because it is impervious to light or moisture
or gases, the manufacturers of food
and drug products soon discovered it to be ideal for wrapping their products. Foods may be kept in contact with it
indefinitely with perfect safety.
Today aluminum foil is used
for wrapping and protecting a wide variety of products—chocolate bars, candy
mints, chewing gum, yeast cakes, cheese, tea, film rolls, soap, cigars and cigarettes,
to mention a few.
Aluminum is also used for
screw caps for jars of vanishing cream, bottles of lotions, and many food and
drug products. Aluminum caps (often in color and bearing printed trademarks or
designs) are used for sealing bottles of ketchup, pickles, salad dressing,
etc., and for capping bottles of proprietary remedies.
Perhaps more interesting
still, soft aluminum is made into collapsible tubes for tooth paste, shaving
cream and numerous pharmaceutical products.
How little did Charles Martin
Hall dream what a contribution he was making to the
comfort of life on that February
morning when he rushed out of his woodshed laboratory with a few globules of
aluminum in his hand and started for Prof. Jewett's laboratory to break the news of his success!
And the
end is not yet.
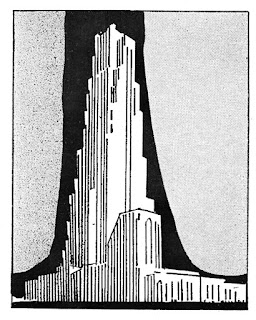
As the
skyscrapers in our great cities have pushed their
towers higher and higher, the
problem of weight has become more
and more important. Much metal is used for decorative purposes— for cornices,
spandrels, window frames, leaders and gutters, and the
like. This has opened up a whole new field for this metal that is only
one-third as heavy as the older
structural metals, will not rust, tarnish or discolor, and can be rolled or
cast or worked into any desired form.
Many modern offices are
furnished with aluminum chairs, either
left in their natural bright finish
or finished to resemble mahogany or walnut. And in many hospitals and
sanitariums and railroad dining cars, aluminum chairs are used because they can be lifted so easily and they will stand all sorts of abuse. Col. Lindbergh's
"flying office" is equipped with aluminum furniture.
Almost every month now a new
use is found for aluminum in some of
its forms or alloys. The business that started in an Oberlin, Ohio,
woodshed now spreads out over the
map of America from Niagara Falls to Bauxite, Arkansas;
and from Edgewater,
New Jersey, to Oakland, California.
In addition to mines, it operates great ore reduction plants, power plants,
foundries, rolling mills, tube mills, wire mills, and a variety of fabricating
plants, employing nearly 25,000 men and women.
And the
Aluminum Age is only in its dawn stage!
All this 'is amazing, when
you stop to think that, until Charles Martin Hall discovered how to produce
aluminum electrolytically in 1886, this metal which now plays so large a part
in our lives, was so expensive that it was regarded almost as a semi-precious
metal! Indeed, within the memory of
some who will read this story, aluminum
sold for $25.00 a pound, whereas it now sells for less than 25 cents a pound,
thanks to this Oberlin, Ohio, school-boy—and to the
activities of the scientists and
engineers of Aluminum Company of
America. Imagine being able to buy a $25.00 rug or a $25.00 chair for less than
25 cents!
Of course, Hall's original
process has been greatly improved, in the
forty-odd years that the research
engineers of the aluminum industry
have been working on this almost magical metal. Hall himself would be amazed at
some of the
properties of the aluminum that is
now being made, and at the enormous
plants producing great aluminum castings and beams and forgings, side by side
with airplane propellers, automobile
parts, paint pigment, aluminum cable, and tissue-thin foil.
You are probably curious to
know whether Hall realized a fortune
from his discovery. So few inventors
do.
Well, Hall did. When he died
in 1914, he was the largest
stockholder in the Company and left an estate worth several millions.
And the fine thing about it was that
he left his stock in the Company to educational and philanthropic causes.
One-half was left to Oberlin and another
American college, one-sixth to education in the
near east and the remainder to
philanthropic organizations.
But his greatest legacy was
an industry which serves us all, making our work easier and our lives more
cheerful and comfortable.
Aluminum Company of America is not the only factor in this fast-growing American
industry. It is the largest single
factor, and the sole producer of the virgin metal in this country. But there are a number of other
important companies making aluminum
castings, sheet aluminum for fabrication purposes, and aluminum products of
many kinds. Furthermore,
considerable foreign-made aluminum is imported.
In order to establish the identity of its own brands, and to build
good-will around its name, Aluminum Company
of America
has created a trade name. That name is made up of three syllables, AL CO A,
standing for the beginning letters
of the principal words of its name:
ALuminum COmpany of America.
ALCOA ALUMINUM is the highest standard in the
world.
To all modern developments
aluminum is making an important contribution. To some
it is absolutely essential. That is why, as we face the
future in America,
we find ourselves only just crossing the
threshold of this wonderful new age—The Aluminum Age!
ALUMINUM COMPANY of AMERICA;
Oliver Bldg. Pittsburgh, PA.







No comments:
Post a Comment